When you need to mix something at a very small scale you don’t reach for a teeny-tiny whisk. If you’re working with microscale biochemical applications you’d be more likely to rely on diffusion to mix fluids. With highly ordered laminar flow there is no turbulence involved, thus making diffusion a prime candidate for “getting the job done”. But what if you need to mix larger molecules? Larger molecules mean higher molecular weight, which in turn leads to very long equilibration times. This is where the electroosmotic micromixer comes into the picture.
When mixing molecules like proteins or peptides for analysis purposes it’s important to be able to reach equilibrium within a reasonable amount of time. While small molecules can take advantage of diffusional mixing thanks to quick diffusion times, heavier molecules cannot. Instead, electroosmotic micromixers should be used. These utilize electroosmosis in order to mix the fluids together. A time-dependent electric field is applied, resulting in electroosmosis. This further leads to parallel streamlines in an otherwise highly ordered laminar flow.
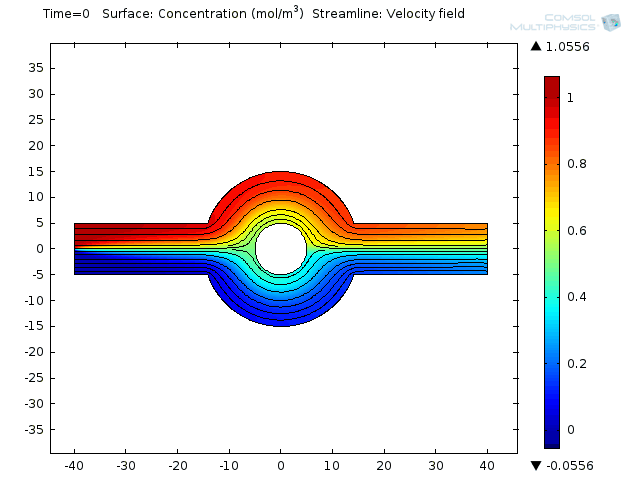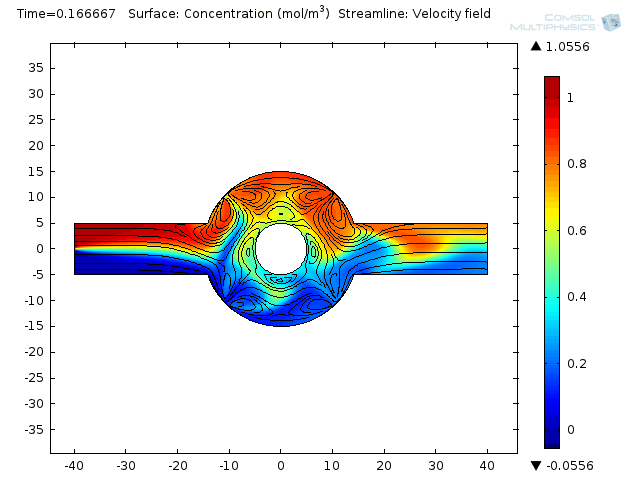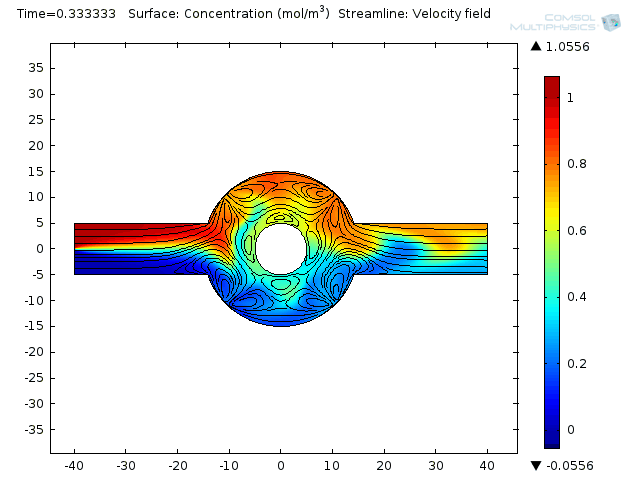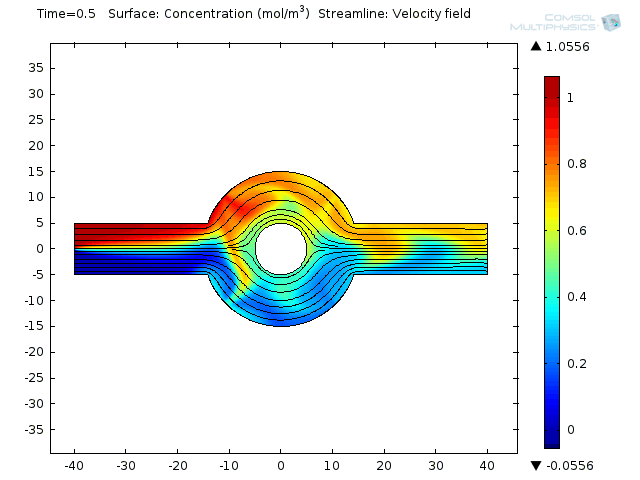
Two fluids enter into a single channel from different inlets leading to a ring-shaped mixing chamber.
Effective mixing involves a combination of repeated stretching and folding of fluid elements and diffusion at small scales. In the electroosmoticmicromixeran alternate current (AC) field is applied, causing the electroosmotic flow to stir up the laminar pressure-driven flow. That brings about moving the combined stream pattern up and down at the beginning of the mixing chamber. Finally, extensive folding and stretching of material lines ensues. Et voilá! Your weighty proteins are mixed.
By the way, there’s also this great3D animation of the electroosmotic mixer.



Comments (0)