Electrochemical Impedance Spectroscopy in Carbonate Buffered Media for Biosensing Applications
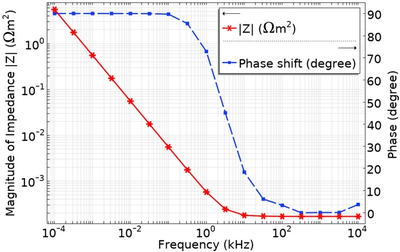
Electrochemical Impedance Spectroscopy (EIS) is a technique commonly used to investigate the properties of the electrode surface and the bulk electrolyte in an electrochemical cell [1]. An AC excitation signal is applied with a frequency range, typically from 0.01 Hz to 1 MHz, and the resulting impedance response, consisting of both real and imaginary parts, is analysed. In an electrolyte, the impedance response at low frequencies (typically below 100 Hz) provides information about the characteristics of the electrode surface, i.e., double layer capacitance, charge-transfer resistance, and diffusion-based impedance, whereas, at higher frequencies (typically in the kHz range), the impedance is primarily governed by the bulk electrolyte resistance [1]. In biosensing applications, EIS technique can be used to detect biomarker by monitoring the electrode-electrolyte interactions as well as the bulk electrolyte properties [2]. To design an EIS electrode for a specific frequency range, it is crucial to have a thorough understanding of the electrode-electrolyte interface and the bulk electrolyte properties. While simplified estimations can be made for electrode geometries such as a parallel plate capacitors and inert electrolytes (those with no bulk or electrode surface reactions) using a simplified Randles circuit, the calculations become cumbersome and inaccurate when dealing with the complex electrode geometries we are considering such as planar interdigitated electrodes and electrolytes with bulk reactions. In such cases, numerical simulation offers a more effective solution. In this work, we present the development of a COMSOL® model to optimize and pre-validate the design of a micro-electrode EIS sensor. The intended application for these electrodes is to measure the EIS response in carbonate buffered media typically used for biological cell perfusion in an organ-on-chip application. Starting with a 1D EIS model of a two-electrode system, we simulate the electrode-electrolyte interaction with a sinusoidal electrode potential excitation and include the bulk reaction of the buffer solution. The Electrochemistry module with 'Tertiary Current Distribution, Nernst-Planck > Tertiary, Electroneutrality' physics interface is utilized, and the AC Impedance technique is employed to study the model. In the AC Impedance study, a frequency-domain perturbation is applied within a range of 10 µHz to 1 MHz. The bulk property of the carbonate buffer solution is incorporated by adding the first order reactions with their defined kinetic rate constants to the physics of the model. The results display the impedance response plotted against different excitation frequencies, allowing the investigation of the influence of electrode design and buffer solution composition. Subsequently, this 1D model will be extended to a 2D and 3D planar interdigitated electrode, as shown in Figure 1, to compare various electrode designs.
下载
- COMSOL-Conference-paper-YA.pdf- 0.88MB
- Abbas_6541_poster.pdf- 3.41MB
