Methanation in Catalytic Reactor
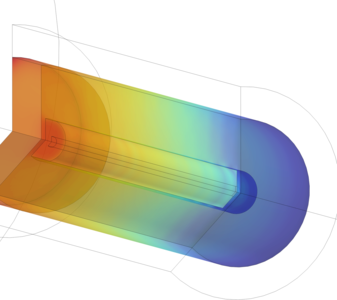
Power-to-gas is an important area of research for reducing greenhouse gases and seasonal energy storage. Methane, with a Lower Heating Value of 50 MJ/kg, is already a commonly used gas, and its synthesis using green hydrogen production (electrolysis of renewable energy) is a remarkable way to break the carbon cycle. Sabatier’s methanation process combines hydrogen and CO2 in a catalytic hot reactor (250-350°C) to produce CH4. CO2 is consumed, and can come from any source, in the present case from a biogas fermenter to produce purely renewable methane. Several constraints influence the efficiency of the reaction: temperature (exo-thermal), pressure (concentration), flow rate (ratio) and cooling (insulation). The target is to maximize CO2 conversion to obtain high-purity CH4 production. The Chemical Reaction Engineering Module, the Heat Transfer Module, the Porous Media Flow Module and the Transport of Concentrated Species interface were used to model two reactors with different configurations. The first design creates a digital twin of the reactor used in the laboratory, while the second is a new design that allows better thermal management. The volume of the reactor is 1.2L and it contains solid catalysts (small cylinders). The temperature distribution in the catalytic bed, the heat generated by the reaction and the gas pressure are obtained, giving a better understanding of how the CH4 conversion works. The results of the digital twin design are used to optimize the design of a better reactor, which will be built and tested in real conditions.
下载
- Schopfer_6461_poster.pdf- 0.89MB
