Modeling Scheil Cooling of a Metal Alloy: Thermodynamic and Multiphysics Solidification
During solidification of a multicomponent liquid, either a metal alloy, a sulphide matte or an oxide slag system, the process is highly dependant not only on temperature but in composition as well. The thermodynamic properties of the system will dictate what phases or mixtures precipitate from the liquid and their compositions, which are different from the original composition of the liquid, thus changing the composition of the remaining liquid that hasn’t solidified. If the solidification process is fast enough so that the system doesn’t have time to equilibrate, then the solid that was created won’t participate on the subsequent solidification step and since the liquid composition has changed, then new phases will form with different composition than the previous solids. This is known as Scheil cooling, or real solidification as it is highly unlikely that the solids will have sufficient time to equilibrate with the remaining liquid before the temperature continues to decrease during a real solidification process. The challenge is that at each time step, the composition of the remaining liquid and the new solids forming is changing, therefore, changing the shape of the “solid fraction” function that is required to model phase change. The new “solid fraction” depends on the thermodynamic properties of the system which in many real cases correspond to a non-ideal chemical solution. In this paper, a combined thermodynamic and multiphysics model was used to simulate this process using COMSOL Multiphysics® software and M4Dlib [1]. A transient heat transfer and fluid flow model in the COMSOL® software was used to model temperature and phase change of a liquid metal alloy, whereas the composition and solid fraction functions were calculated at every time step using M4Dlib, an external library of thermodynamic properties.
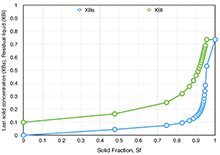
Figure 1 shows the composition of both solid and liquid phases during the solidification process as a function of the cumulative solid fraction for an initial liquid composition of XB = 0.1. The last solid to precipitate has much higher concentration than the original liquid, as opposed to the “equilibrium” solidification case.
The results were compared to three cases: a simple phase change model, the equilibrium solidification case and the analytical solution for Scheil cooling. The comparison between the calculated composition of solid from M4Dlib and the classical Scheil Equation are shown in Figure 2. Finally, the calculated solid fraction function versus temperature is shown in Figure 3, where it is seen that the solidification process starts at the liquidus temperature (Tliquidus = 1227.53K) but finalized at 1053.25K, which is much lower than the solidus temperature for the initial composition of the liquid (Tsolidus = 1176.6K at XB=0.1).
The advantage of using the combined multiphysics and thermodynamic model provided by the COMSOL® software and M4Dlib is that some of the assumptions made by the analytical Scheil cooling equation are not necessarily maintained in a space dependant situation. Finally, the rate of concentration change in the liquid phase is calculated from M4Dlib and can be coupled to a mass transfer model by means of a source term.
下载
- marin_presentation.pdf- 1.05MB
- marin_paper.pdf- 0.62MB
- marin_abstract.pdf- 0.27MB
