Modelling of the Oxygen Consumption of Cells in the Cell Culturing Platform
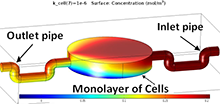
A device for monitoring the oxygen consumption of cells has been developed, which consists of two parts; a cell culturing platform (CCP) and an oxygen sensing chip. The CCP possesses inlet and outlet pipes to direct the fluid under the test to the cell culturing chamber through the inlet pipe and goes out of the outlet pipe after being partially consumed by the cells. In this abstract, the oxygen consumption of the cells in the CCP has been modeled. The electrochemical behavior of the oxygen sensor will be incorporated in this model in future. A cell culturing chamber of cylindrical geometry with diameter of 15mm and 4mm height is designed. The oxygen consumption by a homogeneous monolayer of 10,000 cells inside the chamber is modeled using COMSOL Multiphysics® software. According to the literature, each cell consumes about 10^(-16) (mol/sec) of oxygen [1]. The culture fluid of 200 micro Molar (cO2-in) of dissolved oxygen, which is equilibrated with air, is passing through the pipes and the chamber [2].
In reality without considering the effect of oxygen sensors, the sum of the oxygen consumed by the cells and the output dissolved oxygen of the fluid should be equal to the input dissolved oxygen of the fluid. A laminar inflow condition is considered for the inlet, as a pre-established laminar flow profile.
To model the oxygen transport according to the normal convection-diffusion equation, a coupling of laminar flow and transport of diluted species interfaces has been set, which assumes that the oxygen is very dilute with respect to water. The two interfaces are coupled by using the flow velocity field as the convective velocity field in the convection and the diffusion node. A simple first-order surface reaction for mass loss of oxygen is being set up. The two surface integration are used to compare the rate of inlet to outlet. The flux through the cell layer is set to be oxygen consumption rate (k-cell) by 10,000 cells, 1e-12(m/s) multiplied by the density of oxygen, which is variable in the fluid and evaluated locally and solved for in the transport of diluted species model.
The model is solved with the given data [Table 1] and the diffusional equation as described by Fick’s 2nd law and mesh consists of 50,429 elements. The coefficient of diffusion for oxygen is 3,149x10^(-5) cm/s in the 37℃ water [1]. The reduction of the dissolved oxygen is shown in blue color in the Figure 1. Figure 2 shows the bottom monolayer of cells from the top view.There was a negligible consumption of O2 at the surface, because the reaction rate of kcell was very slow compared to the velocity according to Table 1. A parametric sweep over a logarithmic range of values for this parameter is implemented. For kcell greater than 1e-8 (m/s), an appreciable consumption takes place. At k_cell = 1 (um/s), the consumption of O2 is almost total at some sites. The Slice Plot in Figure 3 illustrates the mass transport gradient.
To verify the reasonableness of this result, one relevant velocity in this system is the ratio of the inlet flow rate to the catalytic area, which is about 1.44e-5(m/s). Significant depletion of O2 cannot be expected if the surface reaction velocity is in orders of magnitude less than this. However reducing the inlet velocity will increase this amount. Therefore by reducing the inlet velocity, there will be more oxygen consumption by cells, which is feasible since the slower flow rate will give more time to cells to react with the fluid and consume more oxygen.
下载
- niazi_poster.pdf - 1.16MB
- niazi_abstract.pdf - 0.58MB
